- Have any questions?
- 0809 992 8864
- info@afrabchem.com
Products

Enaphrin Nasal Drops
March 5, 2019
Amibagyl Suspension
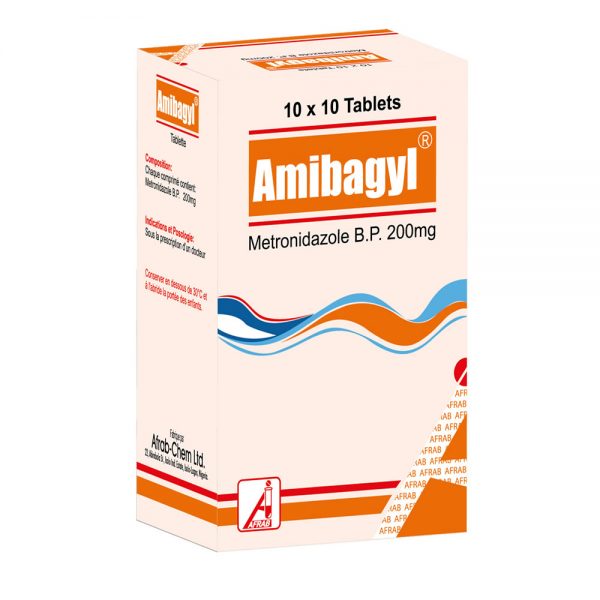
March 5, 2019Amibagyl Tablet
Tablet (NRN: 04-2043): Metronidazole BP 200 mg
How to identify the Product: Off white scored tablet marked ‘AFRAB’.
Pack size: 1000’s; 10 x 10’s
Therapeutic Class
Antibacterial, Nitroimidazole
Dosage Form, Composition & NAFDAC Registration Number (NRN)
Tablet (NRN: 04-2043): Metronidazole BP 200 mg
How to identify the Product: Off white scored tablet marked ‘AFRAB’.
Pack size: 1000’s; 10 x 10’s
Suspension (NRN: 04-0218): Benzoyl Metronidazole equivalent to Metronidazole BP 200 mg per 5 mL.
Pack size: 60ml.
Pharmacology
Metronidazole is a 5-nitroimidazole derivative with activity against anaerobic protozoa and anaerobic bacteria; it also has a radiosensitising effect on hypoxic tumour cells. It is active against several protozoa including Balantidium coli, Blastocystic hominis, Entamoeba histolytica, Giardia intestinalis and Trichomonas vaginalis.
Metronidazole is bactericidal against anaerobic bacteria including Bacteroides and Clostridum species, Gardenerella vaginalis, Campylobacter species and against some Spirochaetes.
Amibagyl acts by penetrating into the cell of the micro-organism and subsequent damage of DNA strands or inhibition of their synthesis.
Indications
Treatment of susceptible protozoal infection and in the prophylaxis and treatment of anaerobic bacterial infection.
The uses are as follows: Bacterial vaginosis (non-specific Vaginitis), Necrotising ulcerative gingivitis (Vincent’s Infection), Giardiasis, Pseudo-membranous colitis, Intestinal amoebiasis, Dracontiasis (Guinea worm infection), Inflammatory bowel disease and as an adjunct to the radiotherapy of malignant neoplasms.
Contra-indications
Metronidazole tablets are contraindicated in patients with a prior history of hypersensitivity to Metronidazole or other nitroimidazole derivatives.
In patients with trichomoniasis, Metronidazole tablets are contraindicated during the first trimester of pregnancy.
Precautions/Warnings
Amibagyl should be given with care in patients with blood dyscrasias or with active disease of the central nervous system. Doses should be reduced in patients with severe liver disease. The use of Amibagyl should be avoided in pregnancy and during lactation.
Interactions
When used in conjuction with alcohol, Metronidazole provokes a disulfiram-like reaction.
It potentiates the activity of oral anticoagulants. Acute psychosis has been associated in concomitant administration of Metronidazole with disulfram.
Adverse Effects
The adverse effects are dose related. The most common are GI disturbances especially nausea and an unpleasant metallic taste. Nausea is sometimes accompanied by headache, anorexia and vomiting.
High doses or prolonged treatment with Amibagyl may lead to peripheral neuropathy usually presenting as numbness or tingling of the extremities, weakness, dizziness, ataxia, drowsiness, insomnia and changes in mood or mental state such as depression.
Dosage & Administration
| Indications | Adults |
Children |
Duration | |
| 1-3 years |
4-10 years |
|||
| Intestinal amoebiasis | 2 – 4 tablets 3 times daily | 2.5-5 mL 4 times daily | 5-10 mL 4 times daily | 5-10 days |
| Bacterial vaginosis |
2 tablets 2 times daily or 10 tablets at once |
– | – | 7 days |
| Anaerobic infections | 2 tablets 3 times daily | 1 tablet 3 times daily | 7 days | |
Storage/Handling Recommendations
Keep in a cool dry place and away from the reach of Children.


Reviews
There are no reviews yet.