- Have any questions?
- 0809 992 8864
- info@afrabchem.com
Products

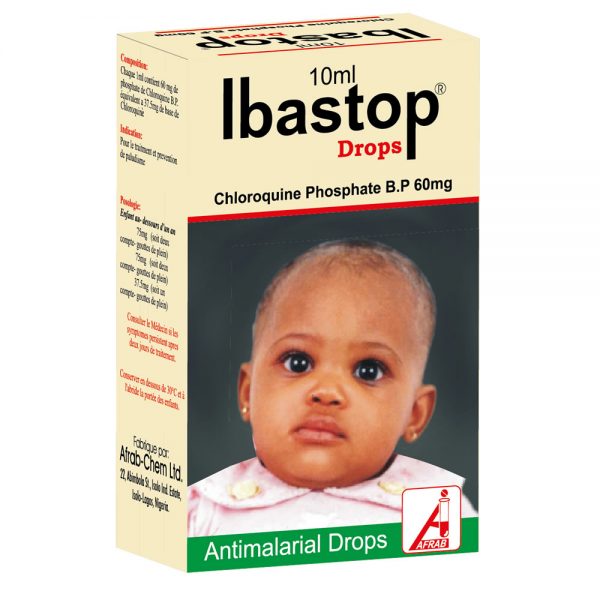
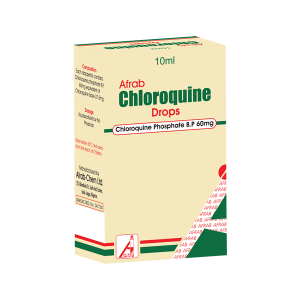
Afrab Chloroquine
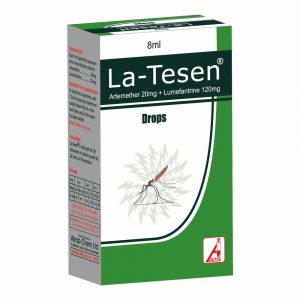
February 28, 2019La-Tesen Suspension
February 28, 2019La-Tesen Tablet
Tablet (NRN: A4-2769): Artemether 40 mg, Lumefantrine 240 mg.
Pack size: Blister pack of 12 tablets
Therapeutic Class
Antimalarial drugs
Dosage Form, Composition & NAFDAC Registration Number (NRN)
Drop (NRN: A4-2045): Artemether 20 mg, Lumefantrine 120 mg.
Pack size: Bottles of 8 mL with dropper.
Suspension (NRN: A4-2044): Artemether 20 mg, Lumefantrine 120 mg.
Pack size: Bottles of 60 mL syrup
Tablet (NRN: A4-2769): Artemether 40 mg, Lumefantrine 240 mg.
Pack size: Blister pack of 12 tablets
Pharmacology
Pharmacodynamics:
LA-TESEN contains a fixed ratio of 1:6 parts of Artemether and Lumefantrine respectively. Artemether is a sesquiterpene lactone derived from the naturally occurring substance artemisinin. Lumefantrine is a synthetic racemic fluorine mixture. Both components of LA-TESEN have their own action site in the malarial parasite. The presence of the endoperoxide bridge in Artemether appears to be essential for antimalarial activity. Morphological changes of the parasitic membranes induced by Artemether have been described, being the result of free-radical action. Lumefantrine interferes more in the polymerization processes.
Other in vitro test suggest that both cause a marked diminution of nucleic acid synthesis. Inhibition of protein synthesis as the basic mechanism of action is suggested in studies which showed morphological changes in ribosomes as well as in the endoplasmic reticulum.
Pharmacokinetics:
Orally administered Artemether is rapidly absorbed reaching therapeutic levels within 60-90 minutes. Artemether is metabolized in the liver to the demethylated derivate dihydroartemisinin. The elimination is rapid, with a T½ of 2-4 hours. Dihydroartemisinin, being a potent anti-malarial itself, has a T½ of about 2-4 hours. The degree of binding to plasma proteins varied markedly according to the species studied. The binding of Artemether with plasma protein in man is about 50%. Radioactivity distribution of Artemether was found to be equal between cells and plasma.
The absorption of Lumefantrine is highly influenced by lipids and food intake. Therefore patients should be encouraged to take the medication with some fatty food as soon as it can be tolerated.
Lumefantrine is N-debutylated in human liver microsomes. This metabolite has 5 to 8 fold higher antiparasitic effects than Lumefantrine. Lumefantrine is found to be highly protein bound (95%). The elimination half life attained in malaria patient will be 4 to 6 days. Lumefantrine and his metabolites are found in bile and faeces.
Indications
LA-TESEN is indicated for the treatment of malaria caused by all forms of Plasmodium including strains from multidrug- resistant areas.
Contra-indications
LA-TESEN is contraindicated in individuals hypersensitive to Artemether and lumefantrine. In patients with a family history of congenital prolongation of the QTc interval or sudden death or with any other clinical condition known to prolong the QTc interval such as patients with a history of symptomatic cardiac arrhythmias, with clinically relevant bradycardia or with severe cardiac disease.
LA-TESEN is also contraindicated in pregnancy especially the first trimester but in view of the high risk of malaria during pregnancy for mother and foetus, it may be considered essential as in the case of cerebral malaria, to treat a pregnant woman. Breast-feeding women should not take LA-TESEN. Due to the long elimination half-life of Lumefantrine, it is recommended that breast-feeding should not start until at least one week after stopping an Artemether / Lumefantrine treatment.
Precautions/Warnings
Interactions
Adverse Effects
LA-TESEN is generally well tolerated by children and adults, with most adverse effects being of mild to moderate severity and duration. Many of the reported events are likely to be related to the underlying malaria and/or to an unsatisfactory response to the treatment rather than to LA-TESEN.
Other common side effects include nausea, vomiting, diarrhea, coughing and trouble of sleeping.
Dosage & Administration
| Body Weight | Day 1 | Day 2 | Day 3 | |||
| Drops | 0 Hrs | 8 Hrs after | Morning | Evening | Morning | Evening |
| 5-10 kg (≤18 months) | 1 mL | 1 mL | 1 mL | 1 mL | 1 mL | 1 mL |
| Suspension | 0 Hrs | 8 Hrs after | Morning | Evening | Morning | Evening |
| 5 | 5 mL | 5 mL | 5 mL | 5 mL | 5 mL | 5 mL |
| 15 | 10 mL | 10 mL | 10 mL | 10 mL | 10 mL | 10 mL |
| 25 | 15 mL | 15 mL | 15 mL | 15 mL | 15 mL | 15 mL |
| Tablets | 0 Hrs | 8 Hrs after | Morning | Evening | Morning | Evening |
| 15 | 1 | 1 | 1 | 1 | 1 | 1 |
| 25 | 1½ | 1½ | 1½ | 1½ | 1½ | 1½ |
| ≥35 kg (Adults) | 2 | 2 | 2 | 2 | 2 | 2 |
Or as directed by the Physician.
Direction for use:
To make 8 mL drops, add 7.5 mL (5 mL + 2.5 mL) of previously boiled and cooled water little at a time and shake until all the water has been added. Shake before every use. Discard the reconstituted suspension after four (4) days.
To make 60 mL suspension add 45 mL or up to the 60 mL mark, of previously boiled and
Cooled water little at a time and shake until all the water has been added. Shake before every use. Discard the reconstituted suspension after four (4) days.
Storage/Handling Recommendations
Store protected from light.
Keep in a cool dry place and away from children.




Reviews
There are no reviews yet.