- Have any questions?
- 0809 992 8864
- info@afrabchem.com
Products

Tussylin Adult Syrup
February 28, 2019
Stopacid Banana Flavour Suspension
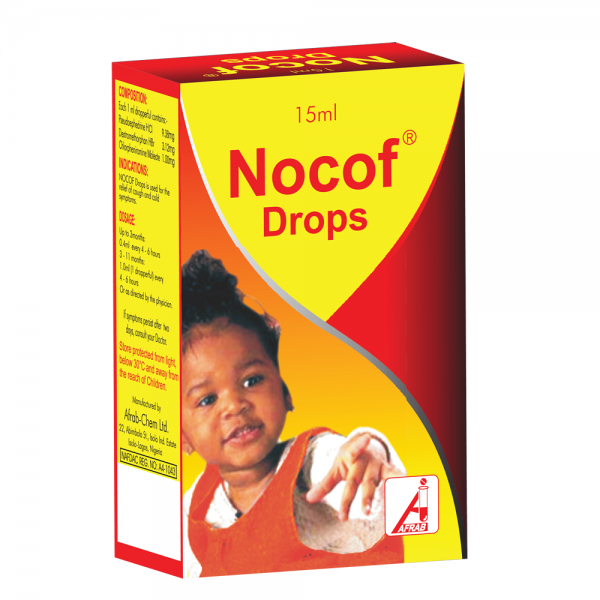
February 28, 2019Nocof Drops
Drop (NRN: A4-1043): Pseudoephedrine HCl 9.36 mg, Dextromethorphan HBr 3.12 mg, Chlorpheniramine maleate 1.00 mg.
Pack size: Bottles of 15 mL with dropper.
Therapeutic Class
Cough and cold preparations
Dosage Form, Composition & NAFDAC Registration Number (NRN)
Drop (NRN: A4-1043): Pseudoephedrine HCl 9.36 mg, Dextromethorphan HBr 3.12 mg, Chlorpheniramine maleate 1.00 mg.
Pack size: Bottles of 15 mL with dropper.
Pharmacology
Nocof drops provide maximum relief for cough, clears nasal congestion to restore free breathing.
Pseudoephedrine hydrochloride is given by mouth for the symptomatic relief of nasal congestion. It is commonly combined with other ingredients in preparations intended for the relief of cough and cold symptoms.
Dextromethorphan Hydrobromide is a non-narcotic cugh suppressant. It acts on cough control in the medulla and raises the threshold of cough. Dextromethorphan has an antitussive activity equivalent to that of codeine. It lacks analgesic and addictive properties and in antitussive doses shows no effects on respiration, the cardiovascular system or the gastrointestinal tract. Dextromethorphan in therapeutic doses does not inhibit ciliary activity and its antitussive effect persists for only 5 to 6 hours.
Chlorpheniramine maleate is a common ingredient of compound preparations for symptomatic treatment of coughs and the common cold.
Indications
Relief of cough, and cold symptoms including running nose and catarrh.
Contra-indications
This product is contra-indicated in patients with chronic cough such as occurs with asthma or if cough is accompanied by excessive phlegm and also in children who have a breathing problem such as chronic bronchitis or have glaucoma.
It is also contra-indicated in patients with or at risk of developing respiratory failure.
Precautions/Warnings
Do not exceed the prescribed or recommended dosage.
Interactions
Should not be taken with alcoholic drinks. However, you should consult with your healthcare provider if you are taking this medication.
Adverse Effects
Side effects include drowsiness, dizziness, dry mouth, blurred vision and gastro-intestinal disturbances. Urinary difficulty and retention, weakness and headache may also occur.
Excitation, confusion and respiratory depression may occur after over dosage.
Dosage & Administration
Up to 3 months: 0.4 mL every 4-6 hours.
3-11 months: 1.0 mL (1 dropperful) every 4-6 hours.
Or as directed by the Physiocian.
Storage/Handling Recommendations
Store protected from light.
Keep in a cool dry place and away from children.



Reviews
There are no reviews yet.